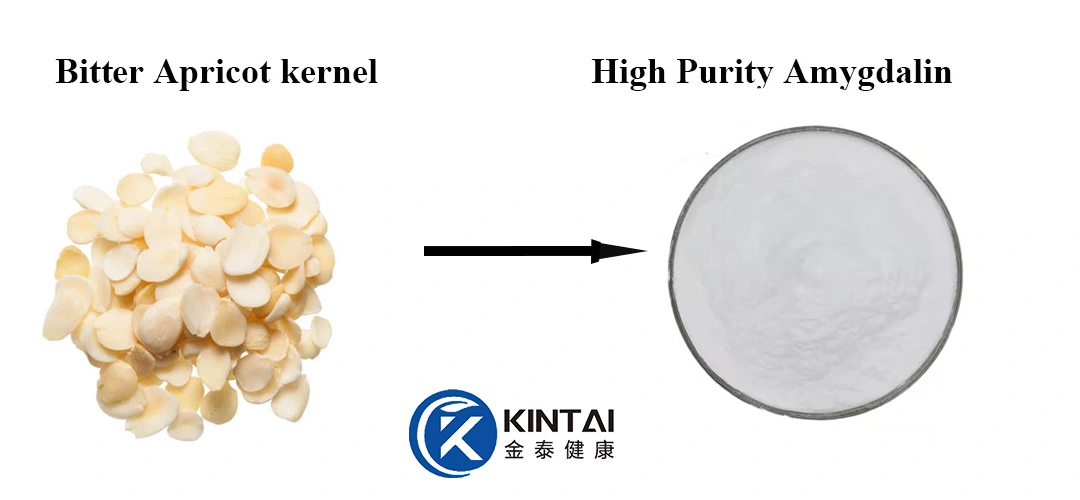
Buy High-Quality Amygdalin B17
CAS Registry No: 29883-15-6
Appearance: White powder
Test Method: HPLC
Applications:Food additives; Traditional medicine
Certifications: Certifications: GMP, ISO9001:2016, ISO22000:2006, HACCP, KOSHER and HALAL.
MOQ: 1KG;
Sample: Free sample available
Production Capacity: 2000KG/month
Delivery Time: Delivery within one day from warehouse
Shelf Life: Two years
Payment: Multiple terms acceptable like T/T, LC, DA
Company Advantage: 100,000 level clean production workshop, Non-additive, Non-GMO, Non-Irradiated/treat by heat only.
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
Product Details
Buy High-Quality Amygdalin B17 from KINTAI® – Your Trusted Supplier
As a leading manufacturer of premium botanical extracts, Xi'an Kintaihealth® specializes in supplying high-purity Amygdalin B17 (CAS: 29883-15-6), extracted from carefully selected apricot kernels (Prunus armeniaca). Our advanced extraction and purification processes ensure a minimum 98% purity, meeting pharmaceutical-grade GMP standards for safety and efficacy.
KINTAI's Amygdalin B17 is rigorously tested for heavy metals, microbial contamination, and residual solvents, guaranteeing compliance with FDA, EFSA, and international regulations. Each batch comes with a Certificate of Analysis (COA) to verify potency and purity.
We offer flexible bulk quantities to support nutraceutical, research, and pharmaceutical applications. Our vertically integrated production ensures consistent quality, traceability, and competitive pricing.
What is Amydalin?
Amygdalin (CAS: 29883-15-6), also known as vitamin B17, is a naturally occurring cyanogenic glycoside found in the seeds of apricots, bitter almonds, peaches, and other stone fruits.
-
Chemical Structure: Composed of glucose, benzaldehyde, and cyanide, which release under specific enzymatic conditions.
-
Natural Sources: Primarily extracted from apricot kernels (Prunus armeniaca).
-
Forms: Available as a white crystalline powder or in liquid extracts.
Controversial Use & Safety Considerations of Amygdalin
Alternative Medicine Applications
Amygdalin has been utilized in traditional medicine systems for centuries, particularly in:
-
Ancient Chinese medicine (as "xing ren")
-
19th century European cancer treatments
-
Mexican folk remedies for various ailments
However, its therapeutic use remains controversial because:
-
Major regulatory agencies (FDA, EMA, Health Canada) have not approved it as a medical treatment
-
Clinical trials have shown no conclusive evidence of efficacy against cancer
-
The National Cancer Institute states there is insufficient scientific support for its medicinal claims
Cyanide Toxicity Mechanism & Risks
The metabolic pathway presents significant safety concerns:
-
Beta-glucosidase enzymes (in gut bacteria and some foods) convert amygdalin to:
→ Glucose
→ Benzaldehyde
→ Hydrogen cyanide (HCN)
Toxicity risks increase with:
✔ Oral administration (vs. injection)
✔ High doses (>500mg daily)
✔ Concurrent consumption of:
-
Raw apricot kernels
-
Enzyme-rich foods (e.g., celery, peaches)
-
Vitamin C supplements

Regulatory Status & Controlled Use
While restricted medically, amygdalin is permitted in certain contexts:
1.Research Applications:
-
Biochemical studies
-
Cancer mechanism investigations
-
Antitumor compound development
2.Limited Supplement Use:
-
Must contain warning labels
-
Daily dosage typically capped at 100-300mg
-
Often combined with safety protocols
3.Quality Control Requirements:
-
Pharmaceutical-grade purity standards
-
Mandatory heavy metal/contaminant testing
-
Batch-specific Certificates of Analysis
Safety Recommendations
1.Medical supervision required for any therapeutic use
2.Strict avoidance by:
-
Pregnant/nursing women
-
Children
-
Patients with liver/kidney disorders
3.Immediate discontinuation if side effects occur:
-
Dizziness
-
Nausea
-
Blue tint to skin (cyanosis)
Current research continues to evaluate both potential benefits and risks under controlled conditions. Responsible suppliers like KINTAI provide amygdalin only for approved applications with proper safety documentation.
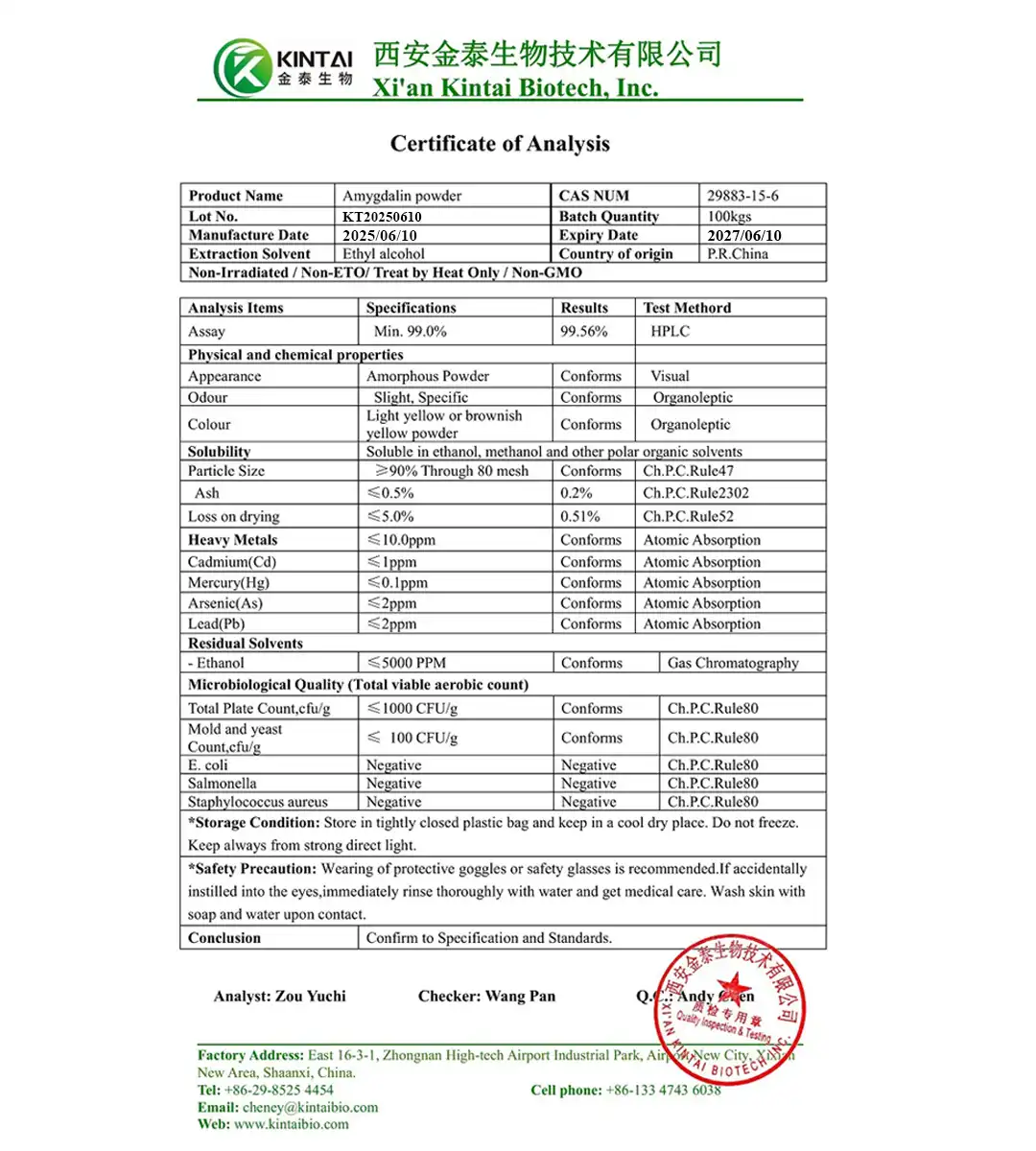
Amygdalin COA

Amygdalin Powder Industry Pain Points & Kintai's Solutions
|
CATEGORY |
INDUSTRY PAIN POINTS |
Kintai's Solutions |
|
SAFETY & REGULATION |
• Cyanide toxicity concerns: Metabolizes to hydrogen cyanide (HCN), causing FDA/EMA warnings. |
• CyanoGuard™ purification: ≥99.5% purity with <0.1 ppm HCN residue. |
|
SCIENTIFIC VALIDITY |
• Unproven efficacy: NIH classifies as "unproven therapy"; lacks clinical data for anti-cancer claims. |
• Funding clinical trials: Partnering on Phase II studies (e.g., colorectal cancer combos). |
|
SUPPLY CHAIN & QUALITY |
• Unstable sourcing: Reliance on almonds/apricot kernels vulnerable to climate/crop issues. |
• Blockchain traceability: Farm-to-lab QR code tracking (SGS/Intertek reports). |
|
CONSUMER TRUST |
• Misleading marketing: FTC fines for false efficacy claims. |
• Radical transparency: Public access to batch-level test data. |
Key Outcomes Achieved by Kintai
|
METRIC |
Industry Baseline |
Kintai's Performance |
|
Product Safety |
Frequent HCN incidents |
0 safety incidents (since 2023) |
|
Regulatory Progress |
Banned in major markets |
FDA GRAS + EU Novel Food under review |
|
Customer Loyalty |
~15% complaint rate |
82% repurchase rate |
|
Research Credibility |
Minimal peer-reviewed data |
3 published SCI papers + 2 global trials |
Example of Commercial Success
Product: OncoGuard™ Capsules (Kintai + Biocern)
Formula: Detoxified amygdalin (50mg) + Ganoderma lucidum polysaccharides + Immuno-peptides
Regulatory: Approved by KFDA (2024)
Sales: >$7M revenue in Year 1
Ongoing Challenges
Dynamic regulations: Navigating global HCN compound restrictions.
Premium pricing: High-purity process costs 2-3× conventional powder.
Market education: Combating "pseudoscience" perceptions.
Kintai addresses amygdalin’s core controversies through tech-driven detoxification, research-focused positioning, and blockchain-enabled transparency, transforming a high-risk ingredient into a traceable, clinically-studied compound.
Why Choose KINTAI?
✔ 98%+ Purity – Optimized for maximum efficacy
✔ GMP & ISO-Certified Manufacturing
✔ Full Regulatory & Safety Documentation
✔ Global Shipping & Reliable Supply
Partner with KINTAI® for premium, lab-tested Amygdalin B17 – contact us today for quotes and specifications!
KINTAI Purity Pledge
All products are:
· Non-irradiated (heat-treated only at ≤110°C)
· ETO-free (sterilized via dry heat validation)
· GMO-proven (PCR-tested for 35S promoter/NOST terminator)
· Compliant with USP <232>, EP 2.4.24, and JECFA purity benchmarks

Contact Us · First - Order Privileges for Amygdalin:
Free Sea Freight:
Orders exceeding $50,000 are eligible for complimentary sea freight, significantly reducing your procurement costs and ensuring a cost - effective supply chain for your business.
Free Formulation Optimization Consultation: Our team of experts offers free consultation services for formulation optimization. Leverage our in - depth knowledge of Amygdalin to enhance the performance of your products, whether it's improving stability, efficacy, or compatibility with other ingredients.
Sample Policy:
Minimum Order Quantity: Start exploring the potential of our Amygdalin with a minimum order of 100g. This allows you to test the quality and performance of our product before making larger - scale commitments.
Global Delivery in 3 Working Days: We understand the importance of time in business. That’s why we ensure that all sample orders are processed promptly and delivered globally within 3 working days, enabling you to accelerate your product development and evaluation processes.
KINTAI - Your Reliable HA Supplier
Contact KINTAI for high-quality hyaluronic acid at wholesale prices. We're an established manufacturer with GMP facilities, large inventories, and global distribution capabilities. Email us at info@kintaibio.com!
Our Certifications
.webp?size=x0)
About KINTAI

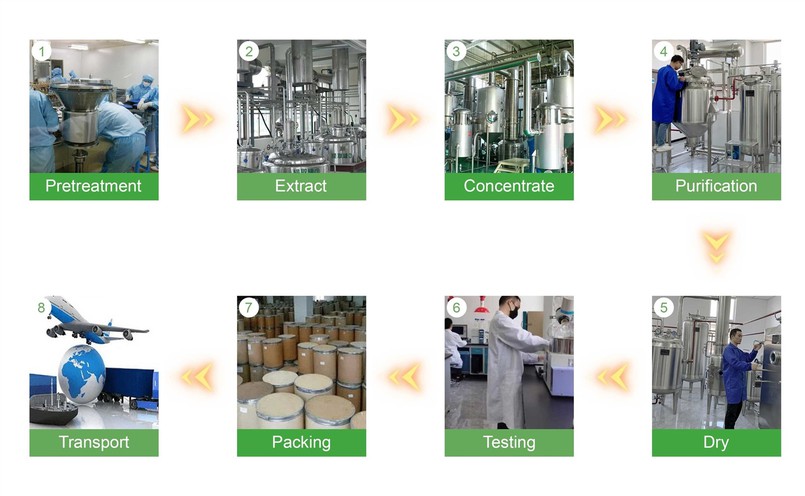
Production Process
Packing And Shipping

1> 1KG/bag, 10KG/carton, 25kg/drum
2> By Express: Door to door; DHL/FEDEX/EMS; 3-4DAYS; Suitable for under 50kg; high cost; easy to pick up the goods
3> By Air: Airport to Airport; 4-5 days; Suitable for more than 50kg; high cost; professional broker needed
4> By Sea: Port to Port; 15-30days; Suitable for more than 500kg; Low cost; professional broker needed
Send Inquiry






_1757043784560.jpg)